PTC has established the Strategic Targeting of Registries and International Database of Excellence (STRIDE) patient registry. STRIDE is an ongoing, multi-center, observational study evaluating the safety and effectiveness of PTC’s novel therapeutic treatment in routine care of patients with nonsense mutation Duchenne muscular dystrophy (nmDMD).
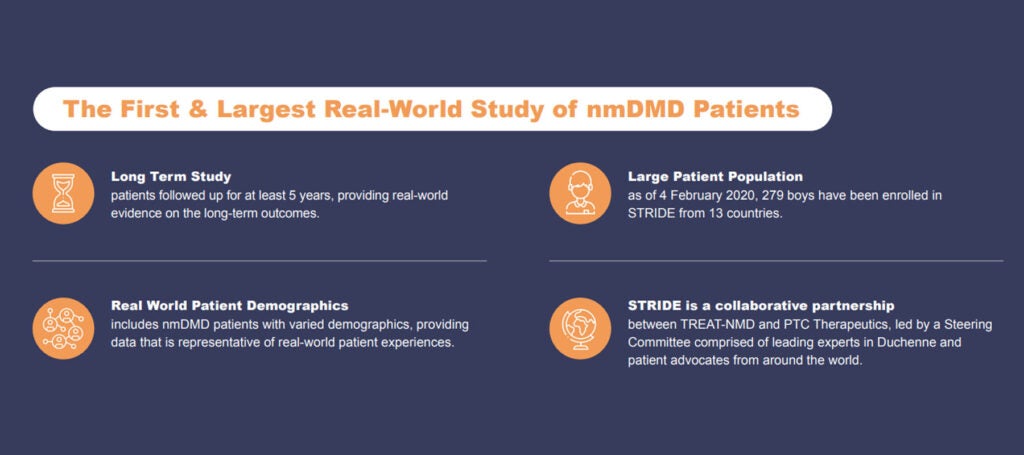
STRIDE is a collaborative partnership between TREAT-NMD and PTC Therapeutics, led by a Steering Committee comprised of leading experts in Duchenne, patient advocates from around the world and PTC representatives. The study includes nmDMD patients with varied demographics, providing data that is representative of real-world patient experiences. Patient enrolment initiated in March 2015 with all patients being followed for a minimum of at least five years, or until withdrawal from the study.
Patient registries serve as an important source of the data needed to assess clinical performance, provide health technology assessment, or assess policy implications on local, regional, national, and international levels. Especially important in rare disease cases that require highly specialised health interventions, registries may be the only means by which data can be obtained.
STRIDE Registry real-world scientific data generated to date has been published in various scientific forums and literature. Insights derived from the study are advancing understanding of this rare disease and answering research questions regarding the prevalence, natural history, and how best to monitor and manage patients. These insights are assisting healthcare professionals in translation of research findings into best clinical practice that supports future activities such as improved standard of care and clinical outcomes for patients. STRIDE scientific data has also served as a strong evidence-based source for regulatory and reimbursement decision-making that supports access to therapy for patients with high unmet medical need.
PTC is committed to improving the lives of those affected by nmDMD. Together with our key stakeholders, we will continue to share the insights and learnings from STRIDE with the broader community through appropriate means.
Our STRIDE Registry was a finalist for a Reuters Events Pharma Awards Europe 2020 award. Congratulations to our award winner, Well Child for Life, and our other finalists in Europe:
- Rare Resolve for Rare Disease – Advancing Diagnosis of AADC Deficiency
- RCPCH – e-Learning for Early NMD Diagnosis in the United Kingdom
- Improving Duchenne Care with Physiotherapy
Our project to accelerate DMD diagnosis in Russia was a finalist for a Reuters Events Pharma Awards USA Global Health Pioneer award in 2020.