Familial partial lipodystrophy (FPL)
What is Familial Partial Lipodystrophy (FPL)?
Lipodystrophies, including familiar partial lipodystrophies, are disorders characterized by a variable loss of adipose tissue (fat), without evidence of weight loss due to lack of food intake or increased energy expenditure, and metabolic complications1.
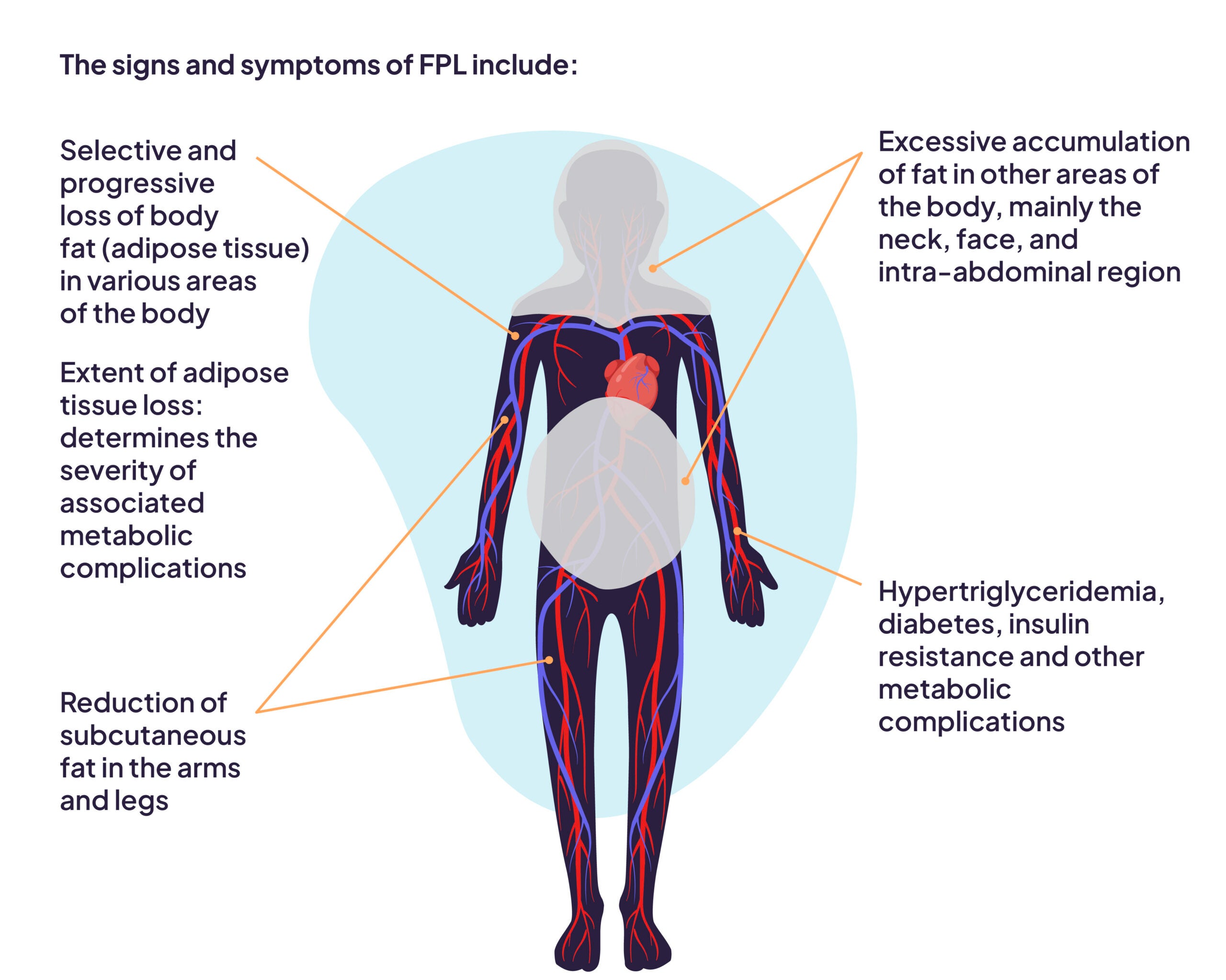
The signs and symptoms of FPL include2:
- Selective and progressive loss of body fat (adipose tissue) in various areas of the body
- Reduction of subcutaneous fat in the arms and legs
- Onset during puberty
- Excessive accumulation of fat in other areas of the body, mainly the neck, face, and intra-abdominal region
- Hypertriglyceridemia
- Insulin resistance
- Diabetes
- Extent of adipose tissue loss: determines the severity of associated metabolic complications
- Other metabolic complications
-
FPL is a highly burdensome disease, leading to significant metabolic complications that are not managed by current therapies, and it is a distressing condition for the patient.
How common is FPL?
The global prevalence of Lipodystrophy is 3.07 cases per million inhabitants, with 2.84 cases per million being Partial Lipodystrophy3.

How is PTC working to treat Familial Partial Lipodystrophy?
PTC has a therapy for FPL that has been in-licensed in Latin America from Akcea Therapeutics, Inc., a wholly owned subsidiary of Ionis Pharmaceuticals, Inc.
[1] Brown RJ, Araujo-Vilar D, Cheung PT, et al. The Diagnosis and Management of Lipodystrophy Syndromes: A Multi-Society Practice Guideline. J Clin Endocrinol Metab. 2016;101(12):4500-4511. doi:10.1210/jc.2016-2466
[2] Zammouri J, Vatier C, Capel E, et al. Molecular and Cellular Bases of Lipodystrophy Syndromes. Front Endocrinol (Lausanne). 2022;12:803189. Published 2022 Jan 3. doi:10.3389/fendo.2021.803189
[3] Chiquette E, Oral EA, Garg A, Araújo-Vilar D, Dhankhar P. Estimating the prevalence of generalized and partial lipodystrophy: findings and challenges. Diabetes Metab Syndr Obes. 2017;10:375-383. Published 2017 Sep 13. doi:10.2147/DMSO.S130810
Do you have questions?
Please reach out if you would like to speak with us.