Duchenne Muscular Dystrophy (DMD)
What is Duchenne Muscular Dystrophy?
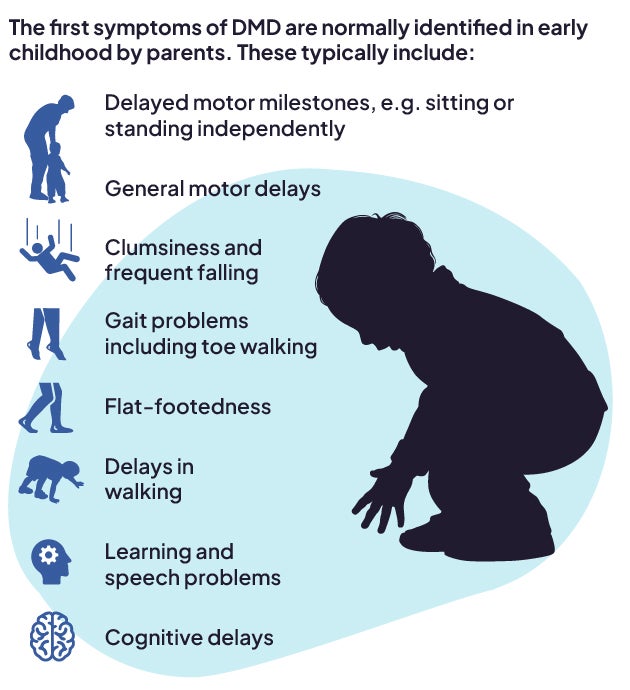
Muscular dystrophies involve progressive muscle wasting and weakness and are caused by a mutation in the DNA that results in either the absence of or very low levels of the dystrophin protein.1 Duchenne muscular dystrophy (DMD) is the most common – and most severe – type of muscular dystrophy.2
Duchenne muscular dystrophy occurs when a mutation in the dystrophin gene prevents the cell from making a functional dystrophin protein.3,4 Dystrophin is a muscle membrane associated protein and is critical to the structural and membrane stability of muscle fibers in the skeletal, diaphragm and heart muscle.3,4 The absence of normally functioning dystrophin results in muscle fragility, such that muscle injury occurs when muscles contract or stretch during normal use.4,5 As muscle damage progresses, connective tissue and fat replace muscle fibers, resulting in inexorable muscle weakness.4,5 Patients with Duchenne muscular dystrophy typically lose the ability to walk by their early teens, require ventilation support in their late teens and, eventually, die due to heart and lung failure.6,7,8,9 The average life expectancy for Duchenne muscular dystrophy patients is in their mid-twenties (mid-thirties with comprehensive health care intervention).10
How Common is Duchenne Muscular Dystrophy? Is it Treatable?

Duchenne muscular dystrophy occurs in approximately one in 5,000 male births and about 20,000 babies worldwide are born with it each year . Genetic tests are available to determine if a patient’s Duchenne muscular dystrophy is caused by a nonsense mutation.10 We estimate that a nonsense mutation is the cause of Duchenne muscular dystrophy in approximately 13% of patients.11
Improvements in the function, quality of life, and longevity of patients with Duchenne muscular dystrophy are often achieved through a multidisciplinary approach to symptom management.6,12,13 With prolonged survival, individuals with DMD face a unique set of challenges related to psychosocial issues and transitions of care, including but not limited to mental health and independence, functionality, and quality of life in critical domains of living, such as education, employment and interpersonal relationships.6,12
How is PTC Working to Treat DMD?
PTC currently has two approved medicines for treating Duchenne muscular dystrophy resulting from a nonsense mutation in the dystrophin gene in ambulatory patients aged 2 years and older: Translarna™ (ataluren) and Emflaza® (deflazacort).6,12
How Can You Stay Informed About Duchenne Muscular Dystrophy?
Resources for Patients and Caregivers
Resources for Healthcare Providers
[1] Theadom A, et al. Neuroepimediology. 2014;43:259-268.
[2] National Institute of Health. What are the types and symptoms of muscular dystrophy (MD)? Available at: https://www.nichd.nih.gov/health/topics/musculardys/conditioninfo/types. [Accessed March 2023]
[3] Goemans N, et al. Eur Neurol Rev. 2014;9:78-82.
[4] Amato AA and Brown RH Jr. Muscular Dystrophies and other muscle diseases. In: Kasper DL, Fauci AS, Hausser SL, et al., eds., Harrison’s Principles of internal Medicine, 19th Ed.
[5] Blake DJ, et al. Physiol Rev. 2002;82:291-329.
[6] Birnkrant DJ, et al Lancet Neurol. 2018;17 445-455.
[7] National Institute of Health. About Duchenne Muscular Dystrophy. Available at: https://www.genome.gov/Genetic-Disorders/Duchenne-Muscular-Dystrophy. [Accessed March 2023]
[8] Mendell JR, Lloyd-Puryear M. Muscle Nerve. 2013;48:21–26.
[9] van Dommelen P, et al. Dev Med Child Neurol. 2020; doi: 10.1111/dmcn.14623.
[10] van Ruiten HJ, et al. Arch Dis Child. 2014;99:1074–1077
[11] Bushby K, et al. Muscle Nerve. 2014 Oct; 50(4): 477–487.
[12] Birnkrant DJ, et al. Lancet Neurol.2018;17:251–267.
[13] Birnkrant DJ, et al. Lancet Neurol. 2018:17:347–361.
[14] PTC Therapeutics. Translarna™ (ataluren) Summary of Product Characteristics. June 2021
[15] PTC Therapeutics. Emflaza™ (deflazacort) Prescribing Information. July 2022
Do you have questions?
Please reach out if you would like to speak with us.